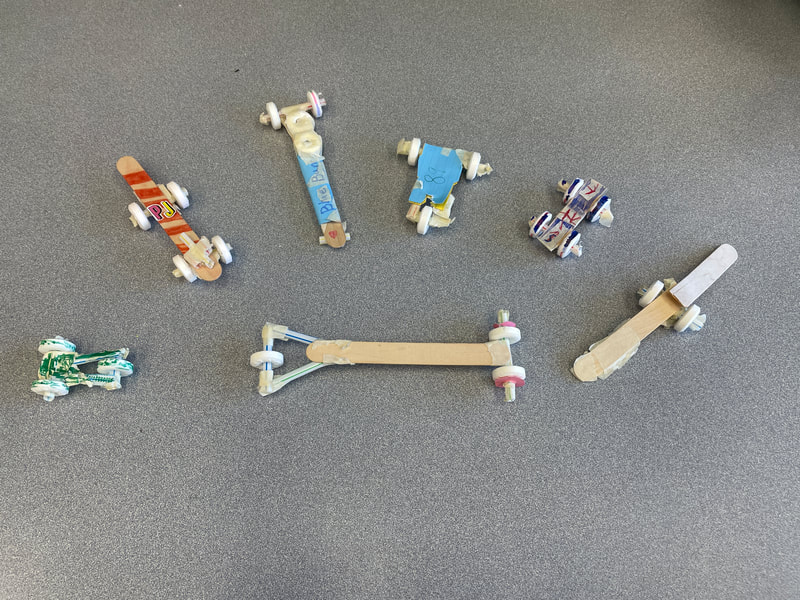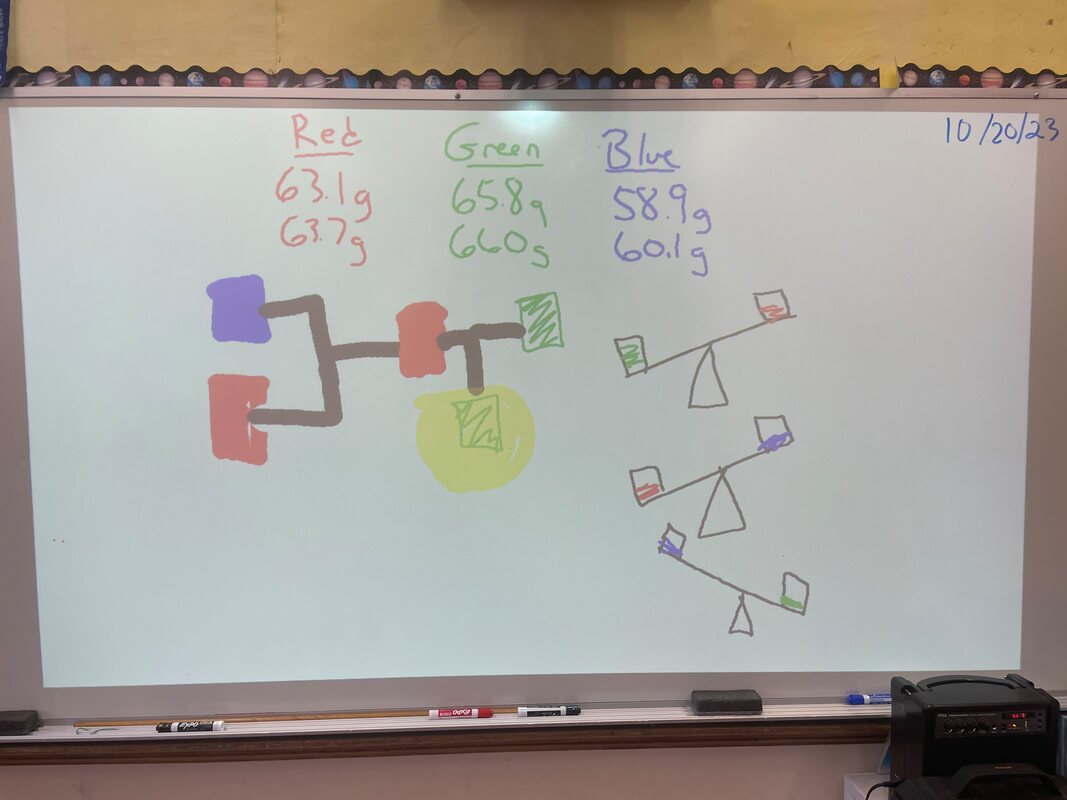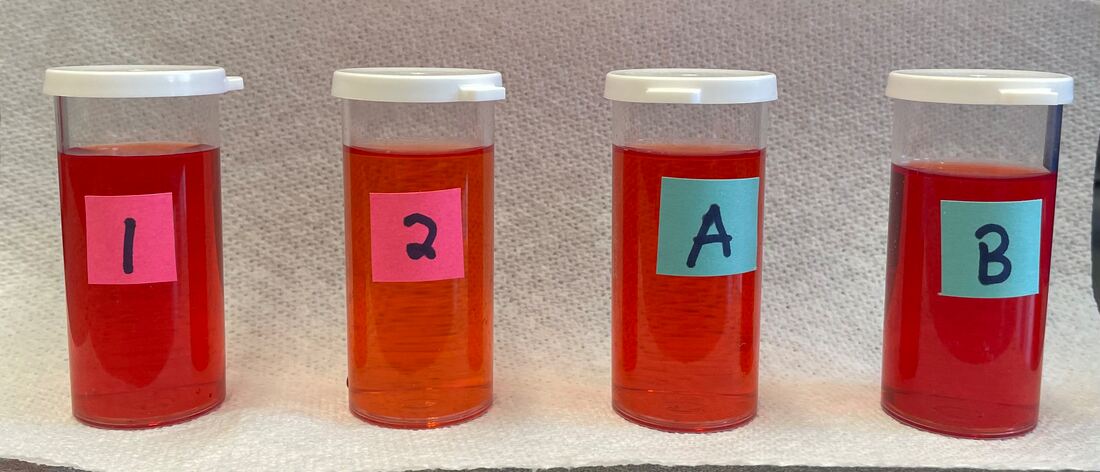Wednesday, May 15th: We closed out our space unit with a very detailed Kahoot! and lots of follow up questions. Tomorrow, we will begin building the country in the social studies.
Friday, May 10th: NJSLA is done! At least until next year. We had a few more solar system presentations and started the documentary Good Night Oppy. We will finish this up on Monday. The documentary is about the rovers Spirit and Opportunity that went to Mars on a 90-day mission. We got our money's worth out of both rovers. Spirit lasted over six years, and Opportunity went even longer, at nearly 15 years.
Thursday, May 9th: Day two of math testing went well. The math assessments are 60 minutes, so they are not overly burdensome or the kids. The kids shared their work and it was creative. Thought-provoking to outlandish would be a good range to encompass their work. The kids said they enjoyed this assignment and it showed in their work.
Wednesday, May 8th: Day one of testing for Math is in the books. Shortened classes were on the menu again, but we made the most of our time working on our solar system creative writing project. Tomorrow, we will share our work with the class and perform any plays or debates.
Tuesday, May 7th: We finished our testing for ELA today and continued with the shortened classes. The kids made good progress on their creative writing pieces. Some of them are very creative. Tomorrow, we will finish up writing and begin to share.
Monday, May 6th: So much for my thoughts on the testing going smoother today. We had a lot of hurry up and wait, but we eventually got to our classes. The kids started a creative writing project. They have a lot of leeway on what they can produce... a story, a play, a group of poems tied to one topic, a graphic novel, a brochure, a debate... Depending on how the testing goes, the kids will have a few days to work on this and share.

Friday, May 3rd: We talked about models throughout the year, and today, we tested it all. We started the day by looking at Ptolemy's geocentric model (Earth at the center), Galileo's heliocentric model (Sun in the center) with only six planets, to modern textbooks, 3D representations, and posters. What did we find out... they are all bad! Today was a once-in-a-lifetime opportunity for the students to create a good model. First, we had to develop the scale. 1 kilometer to 6,000,000 kilometers. Than we did the math. Because of time and age, each group did the math for their planet and one or two more. We then shared the data and confirmed the work. In the end, we had the scale diameters of the Sun/planets and the distance of the bodies from the Sun. We started with the Sun at the end of College Ave (across from the school). We next walked out the planets. 9.7 meters to Mercury. Another 8.3 meters to Venus. A short walk to Earth of 7.0 meters. Onto Mars, another 13 meters from Earth. We finished up at the Montclair State, 750 meters from the Sun (4,500,000,000 km). That put us at the amphitheater. We would have to go much farther to account for the Kuiper Belt (where Pluto lives) and the Oort Cloud. We would have to walk to Relais-Gabriel, Quebec to include the rest of those in our model. Where is that? About 700 miles north of Quebec City.
At the end of this unique learning experience, our students gained a profound understanding of the solar system's vastness, its emptiness, and the fact that the Sun contains a staggering 99.8% of all matter. They also realized the challenges of creating an accurate model, a task they may never encounter again. This understanding deepened their appreciation for the complexity and scale of our solar system.
One last note... with the best possible model the kids will ever see of the solar system... ours could have been better because we had all the planets lined up.
At the end of this unique learning experience, our students gained a profound understanding of the solar system's vastness, its emptiness, and the fact that the Sun contains a staggering 99.8% of all matter. They also realized the challenges of creating an accurate model, a task they may never encounter again. This understanding deepened their appreciation for the complexity and scale of our solar system.
One last note... with the best possible model the kids will ever see of the solar system... ours could have been better because we had all the planets lined up.

Thursday, May 2nd: We did it. We reached the end of our solar system. We closed by learning about the Kuiper Belt and Oort Cloud. The kids have gotten used to understanding distance in AU (astronomical units), so when they found out the Oort cloud is thought to be between 2,000 and 5,000 AU from the Sun, while the outer edge might be 10,000 or even 100,000 AU, they could really understanding of the tremendous size of the solar system.
I am proud of the work the kids did for their presentations. They have shown significant improvement in their research, slide creation, and especially their presentation abilities - confidence, eye contact, clarity, and connecting to the audience). What was even better... kids that were waiting to go, made adjustments or additions during open time to their presentations. They heard what others had missing or what others did well and made those changes.
I am proud of the work the kids did for their presentations. They have shown significant improvement in their research, slide creation, and especially their presentation abilities - confidence, eye contact, clarity, and connecting to the audience). What was even better... kids that were waiting to go, made adjustments or additions during open time to their presentations. They heard what others had missing or what others did well and made those changes.
Wednesday, May 1st: Odd day number two. The kids did better today getting started on the state science test. It was good practice for next week's ELA and Math tests. I mean, they know what they need to do to be ready to start on time. The kids said the test was challenging, and they used most of the time (45 minutes for both sections).
In the shortened class, we continued our presentations on the planets.
In the shortened class, we continued our presentations on the planets.
Tuesday, April 30th: We closed out April with an odd day. The kids had their first taste of NJ state testing for science. We had a rough start (missing headphones, dead or almost dead machines, Wi-Fi issues on one machine), but the kids worked through it and did their best on the assessment. Tomorrow is the last day of testing for science, and it should go much smoother. Next Monday, we will begin the NJSLA testing for math and ELA.
Don't worry, the kids still had all their regular classes. Just shorter. The kids continued their presentations on their planet, and we will finish up by Thursday learning about the Oort Cloud and Kuiper Belt.
Don't worry, the kids still had all their regular classes. Just shorter. The kids continued their presentations on their planet, and we will finish up by Thursday learning about the Oort Cloud and Kuiper Belt.
Monday, April 29th: We made it all the way to Jupiter in most of the classes today. The kids are really starting to understand the immense size of the solar system and how it is teeny tiny compared to the Milky Way and how teeny tiny that is compared to the universe.
Friday, April 26th: Our classes were cut a few minutes short to account for the Bradford Earth Day. It was worth it. The stations were interesting and informational. The kids had a good time. Back in class, we started the presentations. Mercury, Venus, and Earth were presented. The kids had good information and presented well, and we added any missing information. These planets are fascinating once you get to know more about them. Two classes also heard "All Summer in a Day" by Ray Bradbury. This short story shows how little we knew about Venus when he wrote it in 1954. Click here for the story.
Thursday, April 25th: Back to science today, but... we were missing a lot of kids. The kids didn't plan a 5th grade cut day. It was bring your kid to work day. We spent our time going over the Revolutionary War test and started to look at our solar system. First up was the Sun.
Monday, April 22nd: The kids were put into random groups and given a planet to research. They will present their work to the class later this week.
Friday, April 19th: We are back! Not here, but in space. We had a nice opening discussion about what we will accomplish and what the kids already know. It should be an eye opening experience.
Friday, March 15th: It is done! The kids took their unit test today. At first glance, they look pretty good. Come back in a month or so when we explore our solar system.
Tuesday, March 13th: The kids play Kahoot! today for the final review for the test on Friday. Change of plans for Friday's test. The test will now be multiple choice, fill in the blank, and a short essay. The kids voted on the change and I think it will be better. More work for me to create the test in the two three days, but that is part of my job.
Monday, March 11th: I tried some new things today and they worked out great. The first was having the kids in their groups go through their notebooks to review and fill in any missing work. I gave kids copies of notes if they were out on a day. Once the notebooks were in good shape, we tried using a Gimkit. This is new to me, but my daughter at Buzz uses these pretty often. It is a game. I know they already play enough games on computers. The difference is the kids need to answer questions to get energy or snowballs. They need to keep going back to answer questions to keep going. The questions were all based on the Earth System. The kids can use their notebooks for the Test on Friday!
Friday, March 8th: The heavy lifting is done. We completed our atmosphere model. This is pretty much the end of the Earth System unit for science. We have a little more early next week, and then we will spend a day reviewing. The test will be on Friday, March 15th. The kids will be given four prompts, and they need to pick two to answer. They will answer in essay form. Don't worry. They will be ready and able to complete this assessment.
Thursday, March 7th: Atmosphere. It was all about the atmosphere today. The kids were put into groups of two and given a question to research. Next, we got together and started to building a model of the atmosphere. This is a two day project, so the kids will finish tomorrow.
Wednesday, March 6th: We finished discussing the Water Cycle and focused on the distribution of water on Earth. This was half refresher and half new information (going deeper). The kids then created a model of the distribution of water on Earth. Spoiler alert: There is a lot of salt water. But why? Ask your kid. All the water on Earth started as freshwater (from space). Take a look at the model we made.
|
Tuesday, March 5th: The kids presented their water cycle research and completed their models. We took a closer look at condensation. We used ice water and warm water in separate cups. We measured the temperature and moved on to March Book Madness. This contest is for the 4th and 5th graders to pick their favorite book in a bracket-style tournament. We only looked at four contests (eight books) today, but it was great... kids started asking for some of the books they had not read. Back to our cups. The warm water cup had no change, but the ice water cup... lots of moisture on the outside. The kids realized that there is water vapor in the air all around us. It just needs the right conditions to condense. One kid asked if we could make it ran in the room. I told them there is not enough water vapor in here to rain water, but we are always raining knowledge!
|
Monday, March 4th: The next couple of days we will be diving into the Water Cycle. Sure, the kids know evaporation, condensation, and precipitation... but there is more! The kids worked in pairs or alone (the sticks decided the groupings) to become the expert for one part. We started presenting at the end of class and will finish up tomorrow.
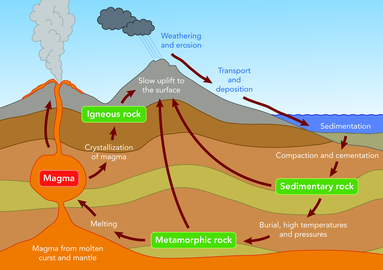
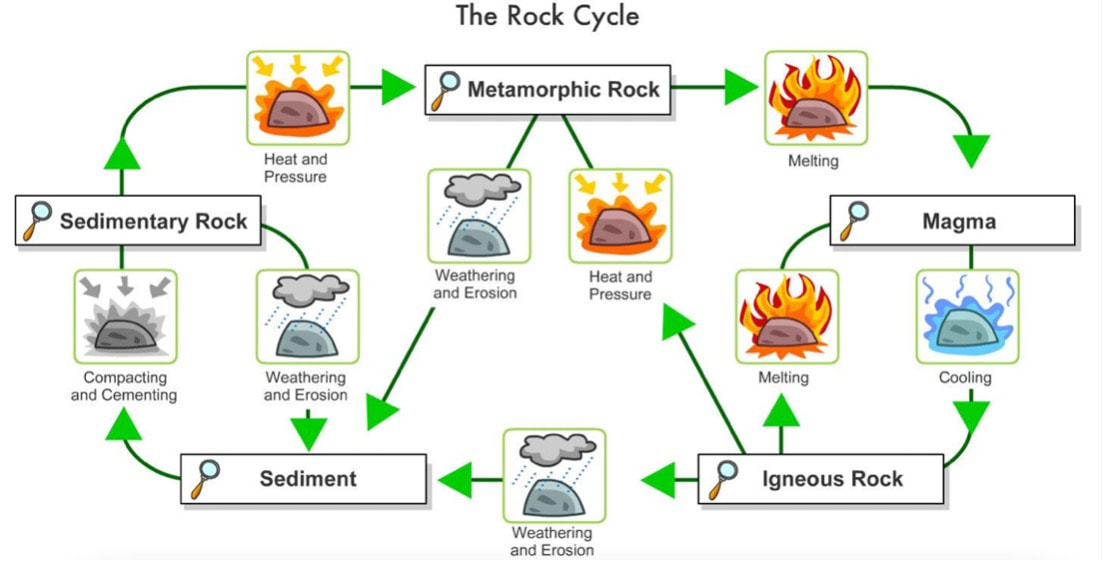
Friday, March 1st: The kids had to escape from a mad scientist's lab. They used their knowledge of the rock cycle and their wits to escape from room to room until they were free! The kids had a lot of fun and reinforced some of the items we have been talking about this week.
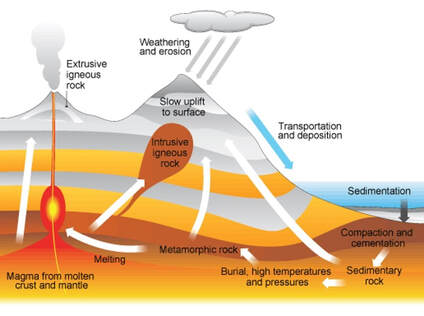
Thursday, February 29th: The kids learned more about rocks than they thought possible. We looked at the rock cycle and defined the terms and processes. We were able to tie the rock cycle to plate tectonics.
Wednesday, February 28th: All the classes finished learning about Plate Tectonics. We spent time using Google Earth to find the plate boundaries by looking for mountain ranges, trenches, volcanoes. I think the kids have a new view of the Earth's surface. There was also a couple of new parody videos. Check them out by clicking on the "VIDEOS" tab.
Tuesday, February 27th: Turns out Alfred Wegener was not a crazy, crazy man (you kid can explain this or check out the video under the "videos" tab). In 1910 he couldn't prove his hypothesis about Continental Drift in his lifetime, he was proved correct once geologist got a better understanding of the broken crust. It all started with the US Navy in the 1960's... Hopefully, your kid can fill in some of the blanks. This planet is truly amazing!
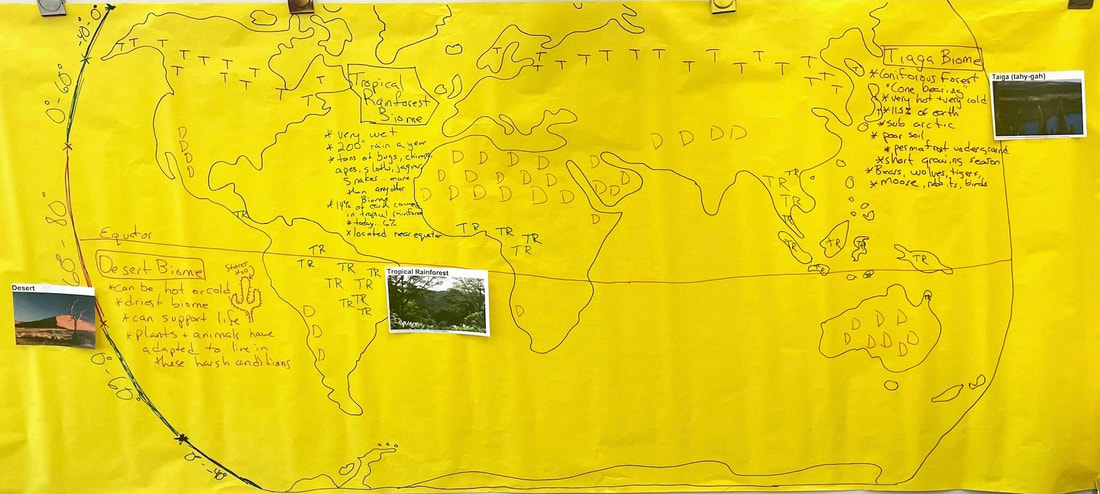
Monday, February 26th: Biome Model... complete. We finished the Biome Model using the kids information that they researched. It came out pretty darn good. The kids made lots of great connections about what is causing differences in biomes and really good generalizations about where they are occurring. An example would be that Tropical Rainforests occur along the equator and near the coast. Great work!
Friday, February 23rd: Biomes. It was all about Biomes today. We began making our World Biome Map based on the the kids research from yesterday. Each kid has a copy of the map below for their notebooks and will keep adding to it on Monday.
|
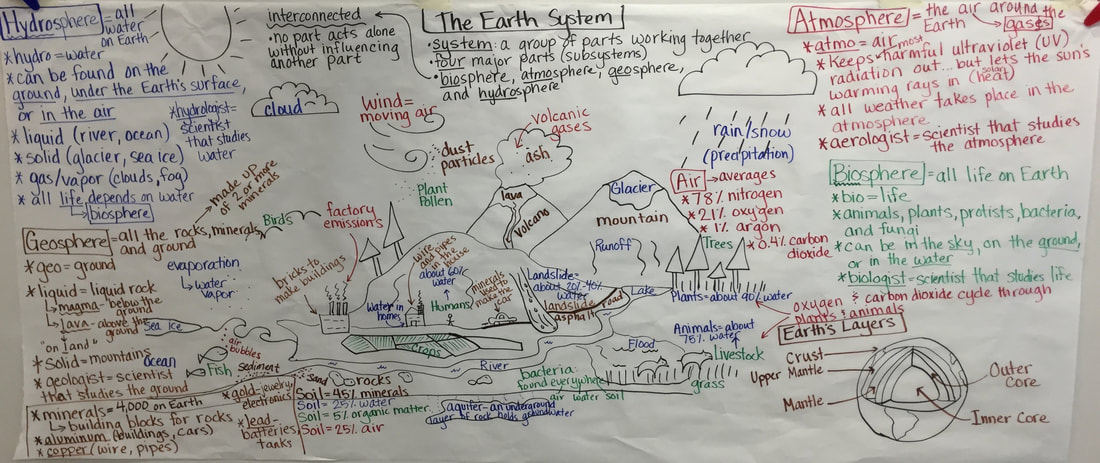
Thursday, February 22nd: We put our knowledge of the Earth System to the test today. The kids looked at the picture to the right and had to find examples of each sphere in the picture. The kids then shared their work with the class. The majority of the class time was spent on researching for our next model... Biomes. Each group of two kids got a biome to research. Tomorrow, they will share what they learned and create a complete biome model as a class. It should be fun!
|
Wednesday, February 21st: Most of the classes finished the Earth's Spheres Model. Over the next couple of days, we will spend time going deeper into each of the spheres... should be fun! Here's our finished work...
Tuesday, February 20th: We began our unit on the the Earth System... four main subsystems... hydrosphere (water), biosphere (life), geosphere (rock) and atmosphere (air). Today's goal: I can develop a model to describe Earth's four major systems. How did we do... not to bad. We got through the background and one system. We will finish the other three tomorrow. The students have the same picture in their notebooks that I have on the board and are adding notes and finding examples. I will include a picture of our model tomorrow. I guide the students, but the information we write down comes from them.
January 11th: The Energy and Matter in Organisms and Ecosystems unit has come to an end. We started the class with a review Kahoot! to refresh the kids memory of the material we covered. Next came an open notebook assessment. If kid's were out on days, they were given access to notes. Mostly, that wasn't necessary because we are constantly referring to previous work throughout the unit.
Come back in a month or so to find out about our exploration of Earth Systems unit.
Come back in a month or so to find out about our exploration of Earth Systems unit.
January 10th: Strange day... part two. We had a shorter class today because of the delayed opening. Shorter amount of time packed with a regular amount of time learning. We finished our time with symbiotic relationships. The kid's Google Slides were shared and a Kahoot! was used to reinforce the information.
January 9th: Strange day. We had an early dismissal because of rain and that made the classes shorter. We will find out tomorrow if this was a good call. Although, I would go with "better safe than sorry". The kids completed their symbiotic project. If they had time left, they worked on their typing for 10 minutes and the rest of the time on IXL ELA.
January 8th: Class started today with an update of the new 3D printers in the building. I spent way to many hours figuring out the machines this weeknd, but finally started getting good prints. We looked at We learned about relationships today. Not the ones that you have to talk about starting in middle school, but symbiotic relationships. We looked at different examples and even heard a couple of parodies. The kids finished the class researching symbiotic relationships in the biome they pulled out of a cup. Ask you kid if they can explain mutualism, commensalism, and parasitism.
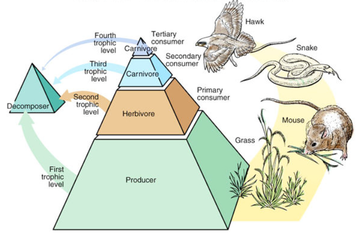
January 5th: Ecosystems. Today was all about ecosystems. The kids learned that an ecosystem is all the living and non-living things that interact with one another in a given area. We watched videos of a desert and coral reef ecosystem and figured out what was biotic and abiotic. Those are a few of the fancy science words we have been using. Another fun set was autotroph and heterotroph. The idea is that the kids will use the correct terminology and not memorize definition and this will allow them a deeper understanding of the material. They will also sound 10% smarter... haha.
January 4th: We finished up our lessons on the Flow of Energy and Matter and discussed the producers, herbivores, carnivores, and omnivores. This is information that the kids already have in their heads. Together, we used that to make connections to our other work done and what is coming up.
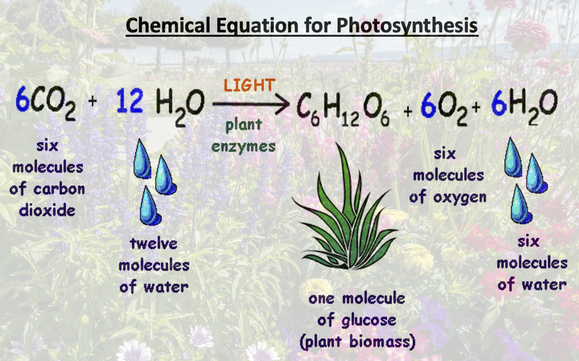
January 3rd: What a waste! We discovered that only 10% of energy is transferred from level to level in a food chain or web. We looked at both of those today. The kids also got a fuller picture of photosynthesis' hows and whys. We even worked on the chemical reaction...
January 2nd: New year... new topic. We started our unit on Matter and Energy in Organisms and Ecosystems. I love this unit because it takes many subjects that the students have learned over the years and connects them. It also connects the kids to everything around them. We started with the basics of energy and matter and how they flow through all living things. We talked a lot about killer whales if it comes up in conversation.
|
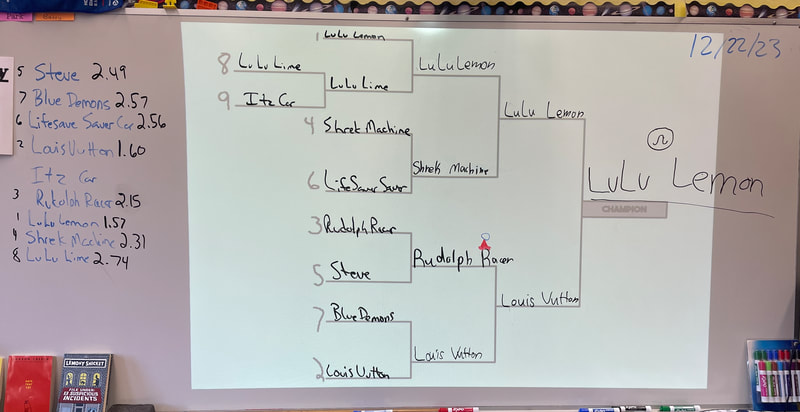
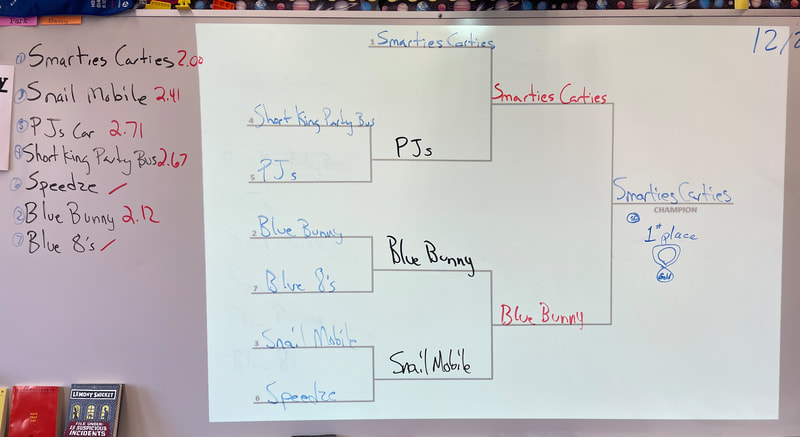
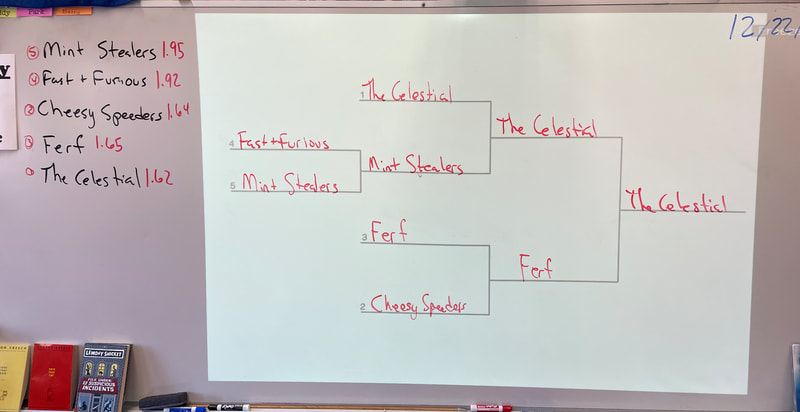
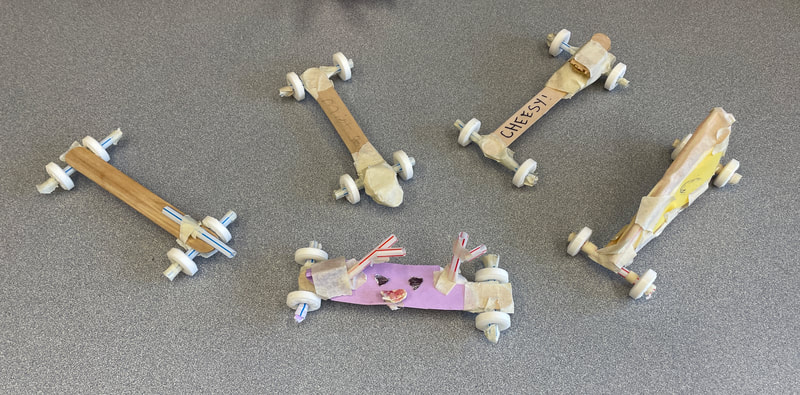
December 22nd: Today is race day! The kids made a few last-minute adjustments, and the races began. We started with a time trial to establish the brackets. You will notice the times on the left next to the car's name. The car had to win two out of three races to move on in the brackets. There was excitement, cheering, disappointment, and fun... a lot of fun.
|
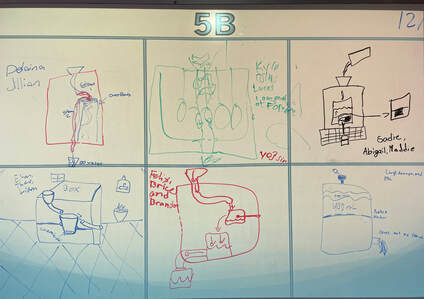
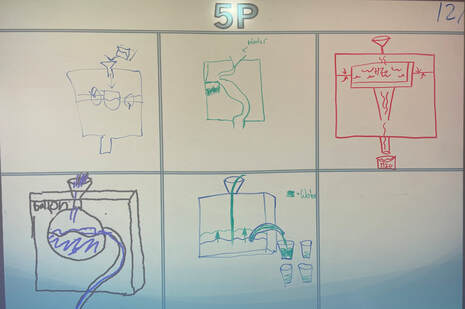
5P
5B
5L
5th Grade showdown of the class winners
December 21st: We paused our engineering project for a special guest speaker. Lily D's dad, Mr. Jacek Dmochowski, came in to talk about his work and passion... the brain. The kids loved his presentation and asked tons of questions. Not all of them were related to what he was asking, but they still showed that they were interested and wanted to know more.
|
December 20th: The concert cut into all the class times, so we had to put of the big race. Might have been for the best, because the extra time today allowed all the groups to refine their cars and a few groups to actually get a running model.
December 19th: How about another engineering project? The kids were tasked with building a car while staying within a budget. They designed, built, tested, and went back to the drawing board multiple times. Tomorrow, they will have time to finish "tuning" up their cars and then we race!
|
December 18th: The kids got a new "black box" or maybe "black bag"... or more accurately a taped shut holiday gift bag. Inside, hidden from the kids view, was a hum-dinger (pull the string and the bag "hums"... let go of the string and the bag "dings")... The students got boards and parts and did their best to create a physical model of what they could not see. They did great... take a look...
|
|
|
|
|
December 15th: What did we do in class today? We created a conceptual model of a Drought Stopper (black box). Although the box was a brown bag that housed the mystery, it is still called a black box. I told the students that if any group could develop a model that matched the black box, I would open it and let them see inside. Some got very close, and with a bit of support, each class was able to figure it out. The best part... some groups came up with unique models that would work. The basic idea inside the box was siphoning, but your kids can explain it better. Here are pictures of their models... no one believed that the black box was "magic".
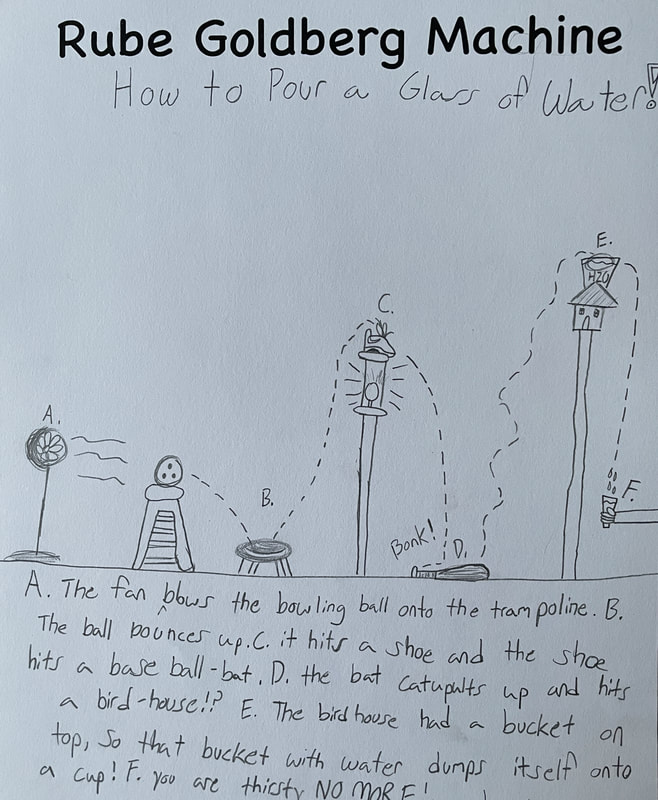
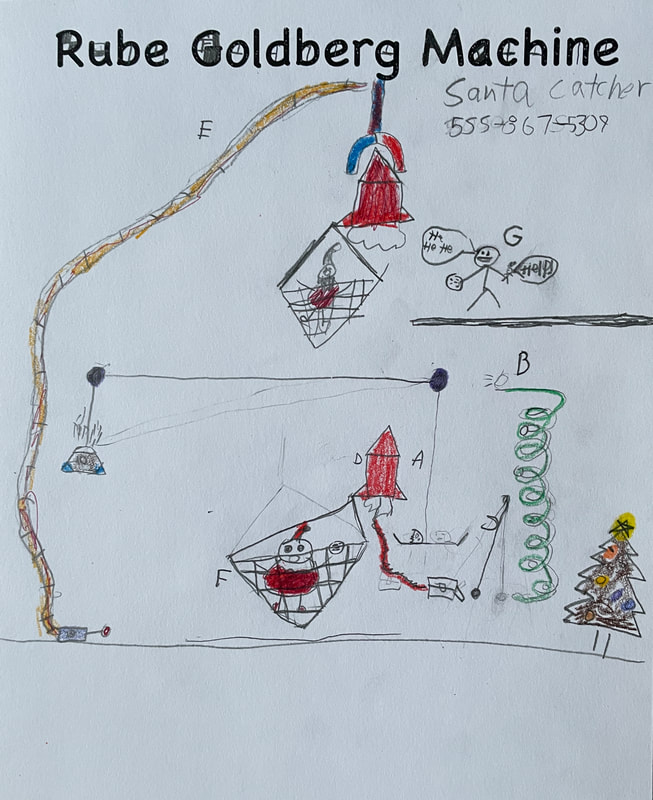
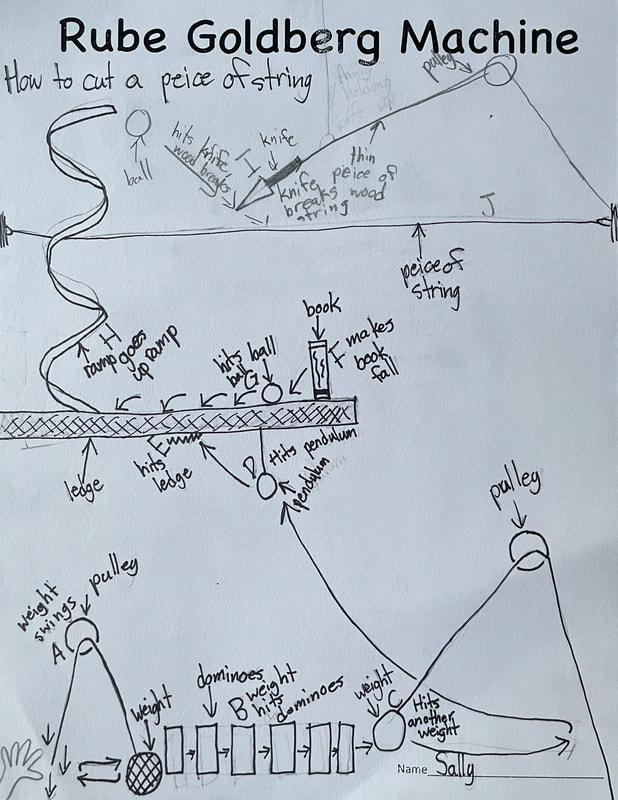
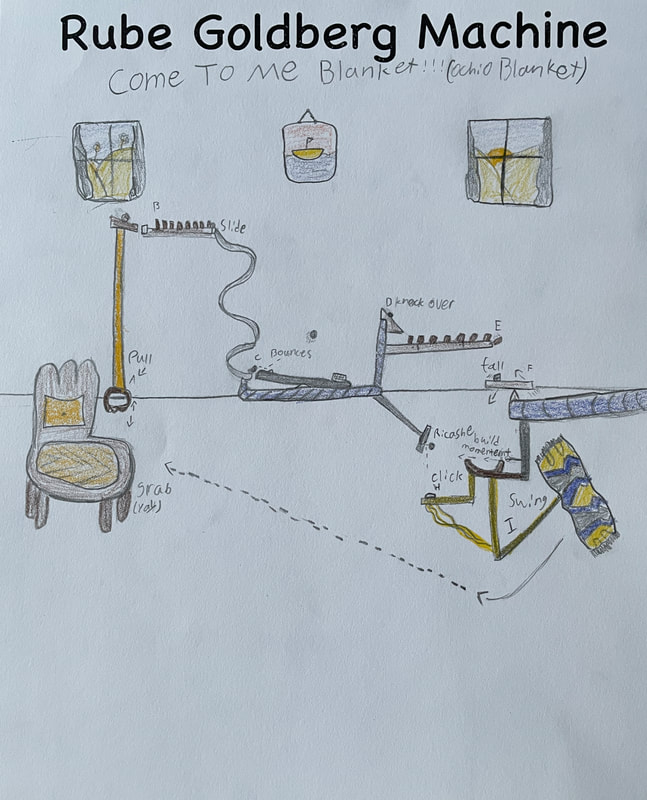
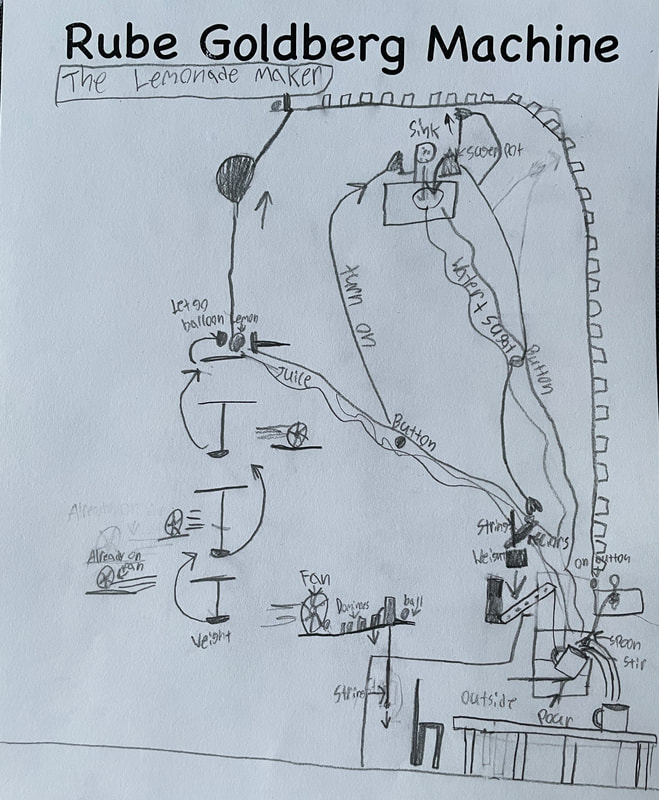
December 14th: Rube Goldberg Machines. Yes, those ridiculous, overly complicated, funny machines that perform a simple task. The kids did a great job of designing their own versions. Here are some samples...
November 3rd: Test Day!!! The tests are not graded yet, but I will have them on Monday for the kids. The quick overview showed that the kids who were following the labs, taking notes, and participating... did well. Some kids struggled, and there is a silver lining. They may realize that what they are doing in class is not working and will make changes.
November 2nd: The kids love to play Kahoot! and it is a great way to review material. We will have our test tomorrow, Friday. They were allowed to take their notebooks home. They don't need to study them or memorize definitions. They could look over the experiments. Most important... they need to bring the notebooks back to school.
November 1st: We made it! Today was our final investigation, and we closed the unit with a bang. Literally... a bang. We captured the carbon dioxide from a chemical reaction in a Ziploc bag. We will start reviewing tomorrow, and depending on how that goes... we may have our test on Friday. If the review does not go as well as expected... we will take the test next week. Don't worry about studying. The kids will use their notebooks for the test, and all the questions come from the experiments/investigations. It will be OK.
October 31st: Happy Halloween. The kids' minds were on the enormous amount of candy waiting for them to collect, but they could still get their schoolwork done and done well. The kids mixed two substances with 50mL of water today. The difference this time was what happened... a chemical reaction. The kids recorded what they observed from each of the three mixtures. It could be temperature change, gasses formed, or precipitate settling out. Sometimes, it was all of them in one cup!
October 30th: Chemical reactions are our final area to cover. We had shortened classes today because of extra gym, but we got some excellent work done and will be ready to hit the ground running with our lab work tomorrow.
October 27th: The kids have reached saturation of the study of saturations. We learned that the mass could identify soluble substances in 50mL of water that has been saturated. They finished the unit by identifying a mystery substance using this method. It turns out the 67g of mystery substance saturates 50mL of water, so of course, it must be citric acid. Next week, we will be investigating chemical reactions and closing the week with a test (it might carry over to the following Monday). We will review prior to the test and the kids will use their notebooks. The answers to all the questions should be in their notebooks. Fingers crossed!
|
October 26th: We know that it takes about 15g of NaCl to saturate 50 mL of water. Is that true for all soluble substances? Epsom salt or magnesium sulfate heptahydrate was today's solute. The groups add one scoop of Epsom salt at a time to 50 mL. They were not surprised that it dissolved in the water. They were surprised at how much was needed to get the solution saturated. The picture to the right shows the saturated solution. You can see the solute that could not dissolve in the solvent and fell to the bottom. The top of the little Post-It Note marked the volume of just 50 mL of water. The Epsom salt added a great deal of volume to the solution.
|
October 25th: How much salt can be dissolved in 50mL of water. That was our project to explore today. The kids used past labs to assist in getting to about 15g by using filters, digital scales, logic, math, and a whole lot of shaking.
|
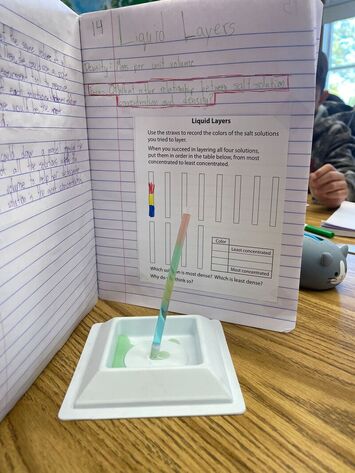
October 24th: We completed the investigation about concentrations. The final lab was the Liquid Layers. The kids were given four different concentrations of salt solutions. They know that density is based on the mass per unit volume, which means that the greater the concentration, the greater the density. They had to stack the liquids in the correct order or the column would mix. It took a bit of work and logic, but they got there.
Can you see the different density layers? Blue is the most dense, so it is on the bottom. That is followed by yellow and green. Finally, the least dense is the red sitting on top. |
|
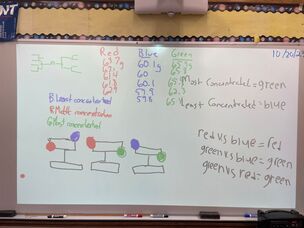
October 20th: We finished the week with mystery solutions. The kids had to work out a procedure to find the most concentrated solution. They had to get the answer from what they learned over the last week. They used balances to get their results and then checked their work on the scales. They did it perfectly!
|
October 19th: We finished our investigation into salt solutions. After much hands-on work, the kids understood the concept that we could determine the more concentrated salt solution by comparing the mass of equal volume. The heavier being the more concentrated. We also went to the Iris Gardens and painted pumpkins.
new concept understood + got out of school and painted pumpkins = a great day!
new concept understood + got out of school and painted pumpkins = a great day!
October 17th: Concentration. Not the game, but the ratio of solvent to solute. Your kid can tell you what those two words mean. Ok, we did have Kool-Aid today, but it was for science. The Kool-Aid or solute was used in different ratios to the water or solvent. We created both dilute and concentrated solutions. The solutions helped them understand the concept and it is Kool-Aid, so they enjoyed the experiment. Today was the exception to the tasting in labs rule. They did not have to drink the Kool-Aid or taste it if they did not want to. If they did, I am sure they would not go wild with sugar, because they had maybe an ounce or two.
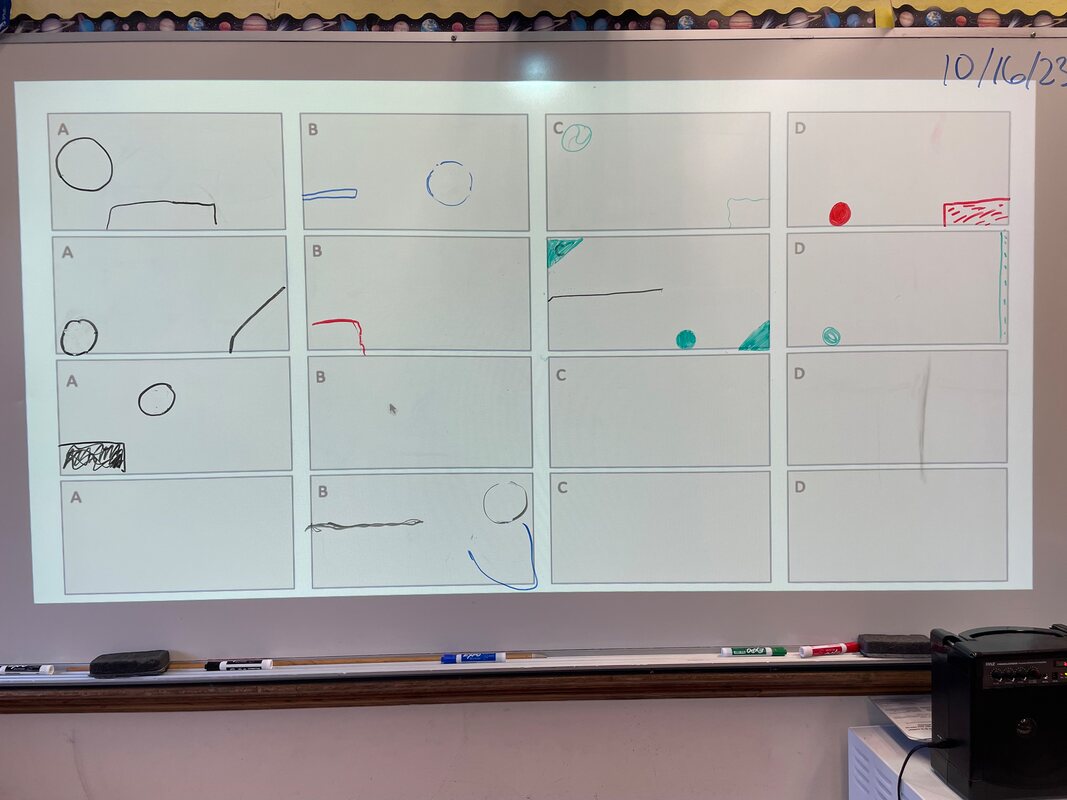
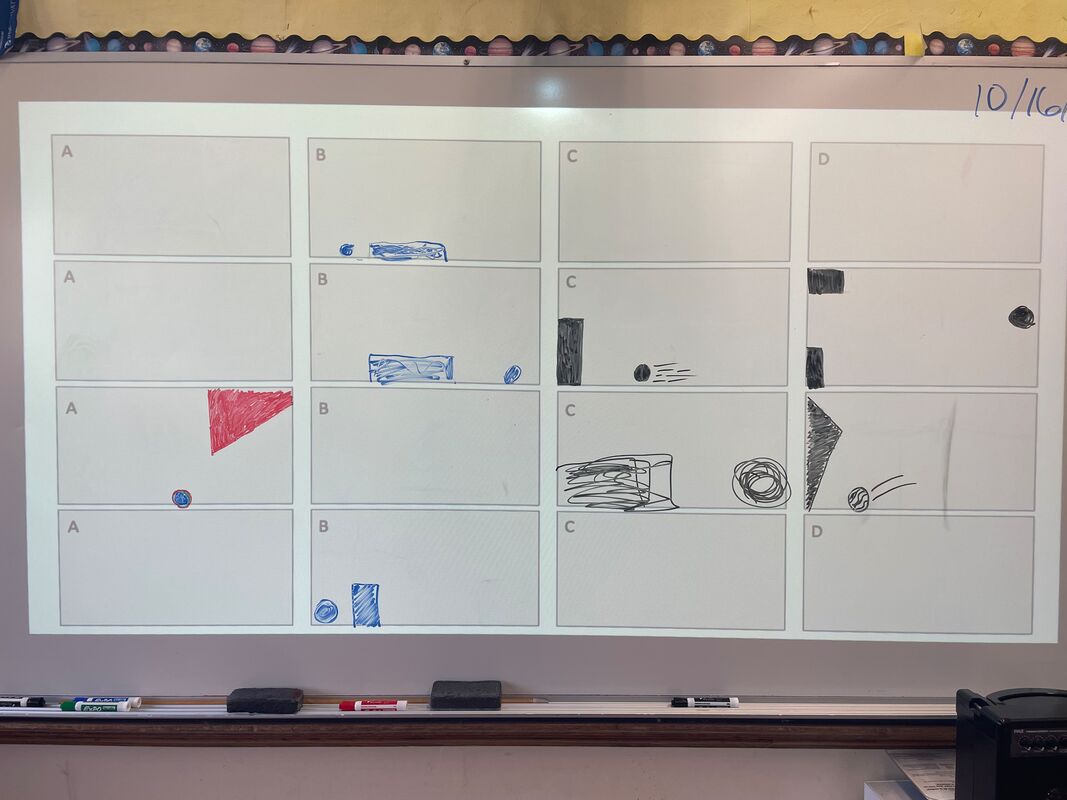
October 16th: The kids got more information about what was in the black boxes. They still could not open their original boxes, but they were able to improve their models. They even got all the possible pieces in their black box to make a model. They did a pretty good job, but because of the nature of the black box... they could not be 100% sure of their work (we can not open the black boxes).
October 13th: Black boxes. That was our topic today. We discussed what is meant by a black box (explanations of objects, events, or systems that cannot be observed directly) and examples (Atomic structure, Earth’s core, Magnetism, and Origin of the universe). Our work centered around a literal black box with something going on inside. The kids did their best to make models of what was in the black box. They made changes as information was shared and ended up with pretty accurate models. It killed them that they could not open the boxes to find out if they were correct... and I didn't tell them. That is what makes them a black box. It was frustrating, but the kids had fun and did some learning.
October 12th: Separating a dry mix was today's lesson. The kids used everything they had done over the last week or so and had to devise a way to completely separate a cup of salt, powder, and gravel. They started by agreeing on a procedure and recording that in their notebook. I checked to see if they had a procedure, not if it would work. Failure is okay in our labs. Quitting is not. The kids couldn't get a new cup of salt, powder, and gravel. They had to figure out how to make it work with what they had... and they did!
October 10th: How do you separate a salt solution? I told the kids that the salt disappears when you mix salt and water. That must be true because I can't see salt in the solution. The kids came up with procedures to prove that the salt was still in the solution by finding the mass of the water and salt and comparing that to the mass of the saltwater solution. Boom! The weights were equal. The solutions are evaporating as we speak, and I hope for much progress tonight. I am working on the students using the correct terminology (solute, solvent, solution, mixture, dissolve...). Tomorrow, we will discuss how the lesson demonstrates the idea of the conservation of matter.
October 6th: The kids finished their first investigation of matter by making and separating mixtures. They had to make observations of their substances, which were Kosher salt, diatomaceous earth, and gravel. The different materials were mixed with water, and the kids had to separate them back out. It was interesting to see their thought processes on what would not work and why and what would work and why. The tricky part was separating the salt that had dissolved (not disappeared) in the water.
October 4th: Today is the beginning of science for the 5th grade. We spent time setting up our notebooks, discussing procedures for lab work, and started to get into the work of understanding matter. I am so excited to open up your children to the wonderful world of science through hands-on experiments.
Check out the tab under Science 2023-2024 called Science World. We have a subscription that comes with online resources. There is a lot there. The articles are excellent, relevant, and tailored to a middle school audience. When you click on the tab you will given the link, username, and password. Take it for a spin. It is awesome.
Check out the tab under Science 2023-2024 called Science World. We have a subscription that comes with online resources. There is a lot there. The articles are excellent, relevant, and tailored to a middle school audience. When you click on the tab you will given the link, username, and password. Take it for a spin. It is awesome.